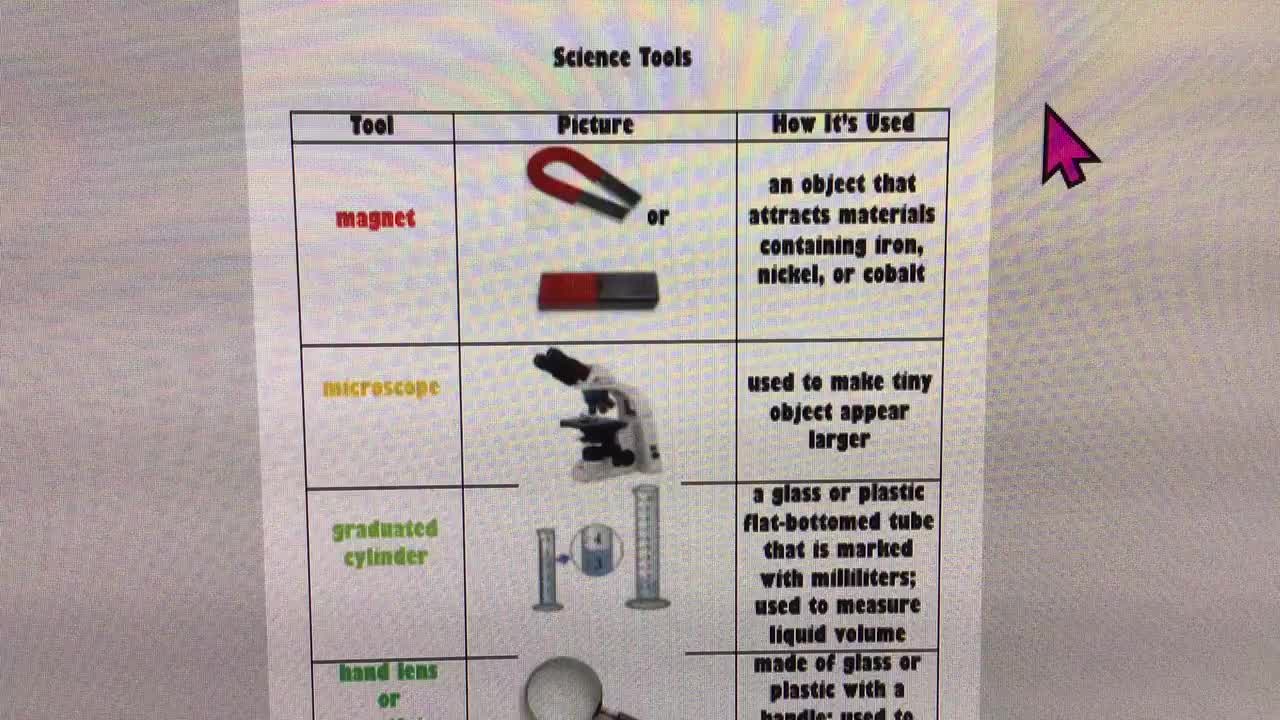
Steve Spangler shows you how to make instant ice using science. This is an excerpt from the 'Fluids' episode of Steve Spangler's TV series DIY Sci. Watch this full episode here: https://xplorationstation.vhx.tv/diy-sci/season:1/videos/104-fluids-episode Subscribe to Xploration Station and receive experiment guides for each experiment of DIY Sci: https://xplorationstation.vhx.tv/diy-sci Purchase DIY Sci: https://xplorationstation.vhx.tv/products/diy-sci-the-entire-collection
Well, water freezes at thirty degrees Fahrenheit, or zero degrees Celsius. But there is this weird thing that happens, and it might have happened to you. You might have left a bottle of water, for example in your car. And you open up the car and you take the bottle of water out and it?s really, really cold outside, it?s well below zero but it?s still a liquid. And as you start to walk away, like magic it turns into a solid. Like this.
And your eyes are not playing a trick man you. It?s really a liquid turning into a solid. And I wanna show you how to do this purposely by supercooling the water.
Start by making an ice bath. Dump ice into water and add rock salt. The salt lowers the temperature below thirty-two degrees by lowering the freezing point. There it is right there, minus ten degrees Celsius. About seventeen degrees Fahrenheit. This is exactly what we need.
Now, add the water bottles. For now, it?s time to just sit and wait. Okay, water, get really cold. Don?t freeze.
After about ten minutes, it?s time. So, if we?re successful and we supercooled the water when we take it out, it will still be a liquid but it?s well below freezing. Now, let?s see if it works. Alright, so here we go. It?s still a liquid. No problem at all. Let?s hit it. Ready, here we go. Bam! It goes fast. Look at it. Here it goes. It?s working its way down. Yes. We are right there. That, that?s solid. Look at that. That is solid. See. There it is.
Try this little trick. Get a piece of ice. Put it on a plate like this. And now you are going to open up one of the bottles. Hoping, crossing your fingers that it doesn?t turn to ice just yet. And then you?re gonna pour it out on to the ice that?s there. Watch this. Well, there it goes. Yes, look at this. Look at this this is perfect look. Bam! That?s cool. Instant ice.
And your eyes are not playing a trick man you. It?s really a liquid turning into a solid. And I wanna show you how to do this purposely by supercooling the water.
Start by making an ice bath. Dump ice into water and add rock salt. The salt lowers the temperature below thirty-two degrees by lowering the freezing point. There it is right there, minus ten degrees Celsius. About seventeen degrees Fahrenheit. This is exactly what we need.
Now, add the water bottles. For now, it?s time to just sit and wait. Okay, water, get really cold. Don?t freeze.
After about ten minutes, it?s time. So, if we?re successful and we supercooled the water when we take it out, it will still be a liquid but it?s well below freezing. Now, let?s see if it works. Alright, so here we go. It?s still a liquid. No problem at all. Let?s hit it. Ready, here we go. Bam! It goes fast. Look at it. Here it goes. It?s working its way down. Yes. We are right there. That, that?s solid. Look at that. That is solid. See. There it is.
Try this little trick. Get a piece of ice. Put it on a plate like this. And now you are going to open up one of the bottles. Hoping, crossing your fingers that it doesn?t turn to ice just yet. And then you?re gonna pour it out on to the ice that?s there. Watch this. Well, there it goes. Yes, look at this. Look at this this is perfect look. Bam! That?s cool. Instant ice.